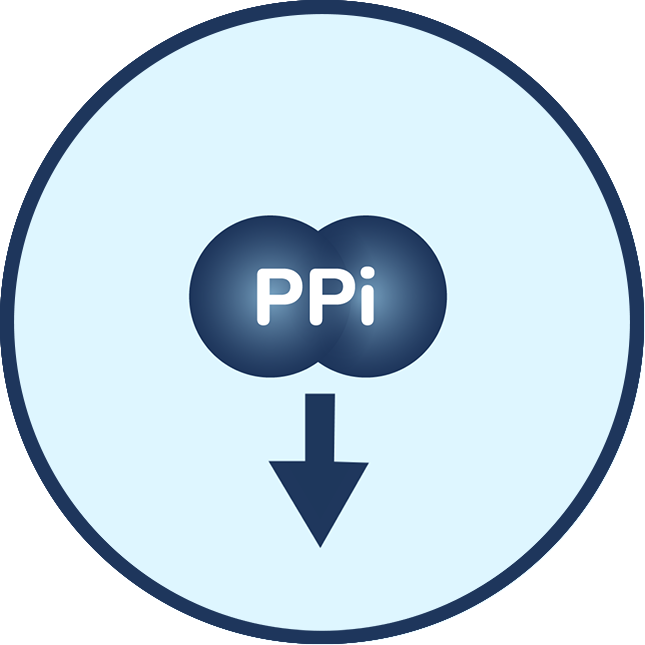
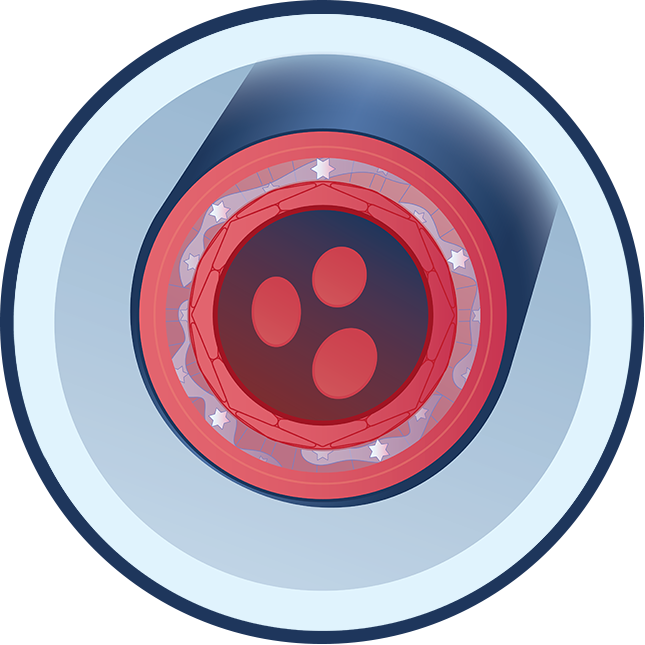
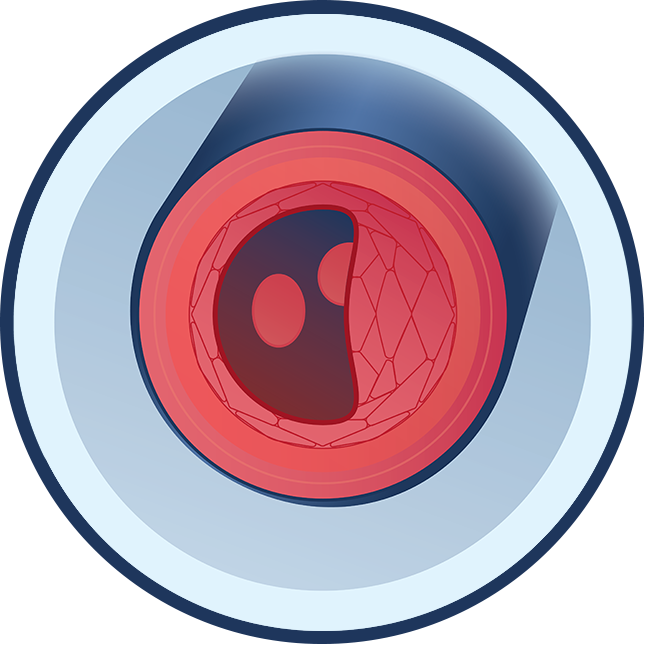
What causes ABCC6 Deficiency?
People with ABCC6 Deficiency have one or two ABCC6 gene variants, which suppresses the body’s ability to produce enough of a protein called ABCC6. ABCC6 Deficiency affects the PPi-Adenosine Pathway by reducing the levels of circulating ATP (adenosine triphosphate) – which is used to generate PPi and adenosine. In ABCC6 Deficiency, the body develops mineral build-up in elastic tissue in the eyes, skin, and vasculature due to low levels of PPi. The body may also have blood vessel narrowing and difficulty maintaining blood vessel health due to low levels of adenosine.